Targeted treatments and antibody therapy
Lots of targeted treatments and antibody therapies are used to treat lymphoma. This information gives an overview of how they work. You may want to use this information to look up a particular type of treatment, rather than reading the whole page.
We have separate information on the individual drugs and on CAR T-cell therapy.
On this page
What are targeted treatments?
Targeted treatments are medicines that have been specially designed to attack particular proteins on lymphoma cells. The proteins they attack are much more common in lymphoma cells than in healthy cells. They are often proteins that are important in helping the cancer cells grow and survive.
Targeted treatments attack lymphoma cells more precisely than chemotherapy. This means they are able to kill lymphoma cells with fewer unwanted effects on healthy cells, leading to fewer side effects.
Some targeted treatments are given on their own. Some targeted treatments are given alongside antibody therapy or chemotherapy.
There are lots of different types of targeted treatment, depending on the particular protein they attack. Many more are being developed in clinical trials.
Types of targeted treatment
There are lots of different types of targeted treatment. They all work in different ways depending on the proteins they attack. Here, we give a brief overview of the main types that are either already available to treat lymphoma, or are in clinical trials for lymphoma. We have more information on the individual drugs, including the exact types of lymphoma they are licensed for – click on the drug name to go straight to it.
Checkpoint inhibitors
Some lymphoma cells are able to hide from your immune system by sticking to a protein on T cells that tells the T cell not to attack. Checkpoint inhibitors block this protein. This means your T cells are able to recognise and attack the lymphoma cells.
Checkpoint inhibitors that are currently available to treat some types of lymphoma are:
B-cell receptor pathway inhibitors
All cells use chemical messengers to send and receive signals to and from other cells. Some of these signals keep the cells alive or make them divide. B-cell receptor pathway inhibitors block these signals in B cells – the cells that grow out of control if you have a B-cell lymphoma. Blocking the signals can make the B cells die or stop them dividing.
There are different types of B-cell receptor pathway inhibitors, depending on the protein they block.
BTK inhibitors
BTK inhibitors block a protein called Bruton’s tyrosine kinase. BTK inhibitors currently available to treat some types of lymphoma are:
Other BTK inhibitors are being tested in clinical trials.
PI3K inhibitors
PI3K inhibitors block a protein called phosphatidylinositol 3-kinase. PI3K inhibitors currently available to treat some types of lymphoma are:
Other PI3K inhibitors are being tested in clinical trials.
mTOR inhibitors
mTOR inhibitors block a protein called mammalian target of rapamycin. At the time of writing, the following mTOR inhibitor is licensed to treat mantle cell lymphoma, although it is not currently available on the NHS:
- temsirolimus.
BCL-2 inhibitors
Cells usually have a natural lifespan. When they get old, they die and are replaced by new cells. This process is usually kept carefully in balance.
B-cell lymphoma-2 (BCL-2) is a protein that can block the natural process that makes cells die when they should. Some lymphoma cells make a lot more BCL-2 than healthy cells, which helps them stay alive longer than they should. BCL-2 inhibitors block the BCL-2 protein. This activates the process of natural cell death.
At the time of writing, the following BCL-2 inhibitor is licensed to treat some types of lymphoma:
Proteasome inhibitors
Proteasomes are chemicals that break down proteins inside cells and recycle them. This helps keep the proteins in balance so the cell works as it should. It is a natural process – but it is especially important for lymphoma cells because they make a lot more protein than healthy cells. Proteasome inhibitors block proteasomes, which leads to a build-up of proteins in the lymphoma cells. The cells are no longer able to work properly and die.
At the time of writing, the following proteasome inhibitor is licensed to treat some types of lymphoma:
Immunomodulators
Immunomodulators are drugs that alter the way your immune system responds to lymphoma cells, helping it work more effectively. They also act directly on lymphoma cells to stop the cells dividing, and activate processes that kill the cells.
At the time of writing, the following immunomodulator is licensed to treat some types of lymphoma:
HDAC inhibitors
HDAC inhibitors block an enzyme called histone deacetylase. This alters the activity of genes to make cancer cells die. HDAC inhibitors also help your immune system respond better to cancer cells.
At the time of writing, there aren’t any HDAC inhibitors licensed for lymphoma, but several are being tested in clinical trials. These include:
- romidepsin
- vorinostat.
What is antibody therapy?
Antibody therapy is a type of targeted treatment.
Antibodies are specialised proteins that your white blood cells make. They help fight infections by sticking to proteins on the surface of cells that don’t belong in your body, such as viruses and bacteria. Once they have stuck to these proteins, the antibodies either kill the cell directly or help your immune system find it and destroy it.
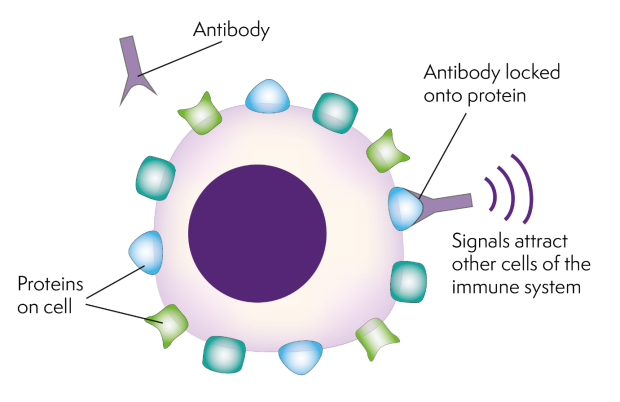
Antibody therapy uses antibodies that have been specially made in a lab. They are designed to recognise and stick to a protein on a cancer cell. This activates your immune system to destroy the cancer cell. Antibody therapy is also known as ‘immunotherapy’ because it works through your own immune system.
Antibody therapy is often given alongside chemotherapy. This is called ‘chemoimmunotherapy’ (or CIT).
Types of antibody therapy
Different types of antibody therapy are available to treat lymphoma. They work in different ways depending on how they are made and the proteins they target. Here, we give a brief overview of the main types that are either already available to treat lymphoma, or are in clinical trials for lymphoma. We have more information on the individual drugs, including the exact types of lymphoma they are licensed for – click on the drug name to go straight to it.
Monoclonal antibodies
‘Monoclonal’ antibodies means that all the antibodies are exactly the same, so they stick to exactly the same protein. When they stick to their target protein, they activate your immune system to destroy the cell that’s making the protein.
There are different monoclonal antibodies available to treat lymphoma that target different proteins. These include:
- mogamulizumab, which sticks to a protein called CCR4 on T-cell lymphomas
- obinutuzumab, which sticks to a protein called CD20 on B-cell lymphomas
- rituximab, which sticks to a protein called CD20 on B-cell lymphomas
- tafasitamab, which sticks to a protein called CD19 on B-cell lymphomas.
Antibody–drug conjugates
Antibody–drug conjugates are monoclonal antibodies joined to a chemotherapy drug. The antibody is designed to stick to a protein on lymphoma cells and carry the chemotherapy drug directly to them. At the time of writing, the following antibody–drug conjugates are licensed to treat some types of lymphoma:
Radio-immunotherapy
Radio-immunotherapies (RIT) are monoclonal antibodies joined to a radioactive particle. They are also known as radioligands. The antibody is designed to stick to a protein on lymphoma cells and carry the radioactive particle to them. This delivers a small dose of radiotherapy directly to the lymphoma cells. At the time of writing, the following radio-immunotherapy is licensed to treat some types of lymphoma, although it is not currently available on the NHS:
- 90Y-ibritumomab tiuxetan.
Other radio-immunotherapies are being tested in clinical trials.
Bispecific antibodies
Most antibody therapies stick to one target protein. Bispecific antibodies stick to two different target proteins – one on lymphoma cells and one on healthy T cells (immune cells that can kill other cells). This helps the T cells find and destroy the lymphoma cells.
At the time of writing, the following bispecific antibodies are licensed to treat some types of lymphoma:
Several others are being tested in clinical trials, these include:
- blinatumomab
- mosunetuzumab
- odronextamab.
