Positive early results for CAR T-cell therapy in mantle cell lymphoma
Published on: 14 May 2020Clinical trial results published.
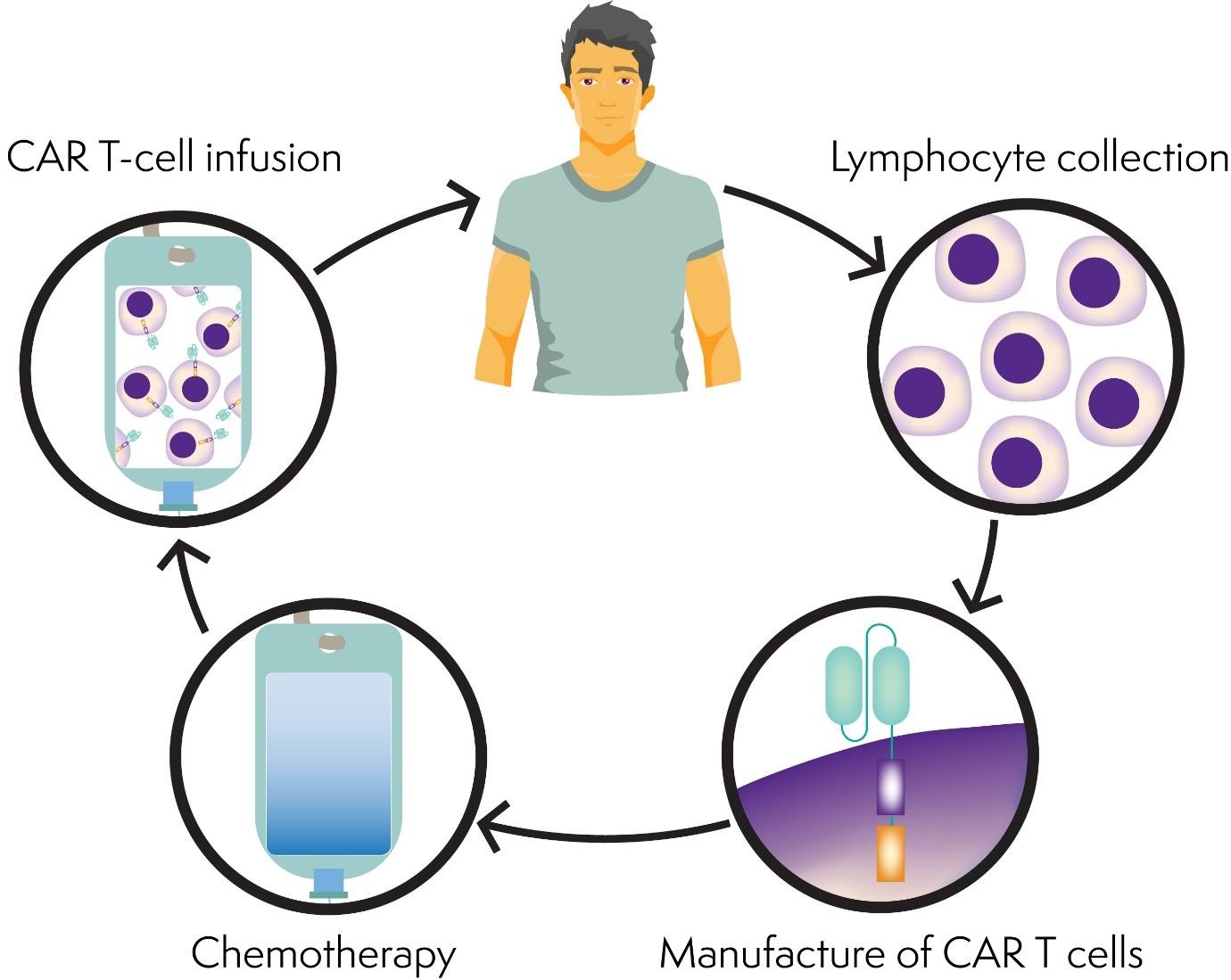
The New England Journal of Medicine has this month published the results of a phase 2 clinical trial of a new CAR T-cell therapy called KTE-X19 in people with mantle cell lymphoma that has come back (relapsed) or not responded (refractory) to previous treatment.
Mantle cell lymphoma is a fast-growing type of lymphoma. Although it usually responds well to initial treatment, it often comes back and is difficult to cure.
CAR T-cell therapy involves having your own T cells collected and genetically modified (changed) in a laboratory to help them recognise and kill lymphoma cells. The modified T cells are then given back to you, like a blood transfusion.
CAR T-cell therapies called tisagenlecleucel and axicabtagene ciloleucel are already available for some people with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B cell lymphoma. This study investigated a new CAR T-cell therapy called KTE-X19 in people with mantle cell lymphoma who had received up to five previous treatments, including a type of targeted drug called a BTK-inhibitor (for example, ibrutinib).
Almost all (93%) people treated in the trial responded to CAR T-cell therapy. Around two in three people had a complete response. After a year of follow-up, over half the people treated were still in remission. However, in common with other CAR T-cell therapies, the treatment can cause serious and sometimes life-threatening side effects. CAR T-cell therapies already in use are only given in specialist hospitals that have the facilities and staff to treat these side effects effectively.
KTE-X19 could be a promising treatment option for people with relapsed or refractory mantle cell lymphoma. It is being studied in further clinical trials and is currently going through the drug approval process in both Europe and the US.
14 May 2020
