First CAR T-cell therapies recommended for approval in Europe
Published on: 29 June 2018These therapies offer a new treatment option for people with difficult-to-treat B cell lymphomas.
Today 29 June 2018 the European Medicines Agency (EMA) announced that two different CAR T-cell therapies are being recommended for approval for people with lymphoma:
- Yescarta (axicabtagene ciloleucel) is recommended as a treatment option for people with diffuse large B-cell lymphoma (DLBCL) or primary mediastinal large B cell lymphoma (PMBL) who have already had at least two courses of treatment but who need more treatment.
- Kymriah (tisagenlecleucel) is recommended as a treatment option for people with DLBCL who have already had at least two courses of treatment but who need more treatment.
This innovative new type of treatment has been a key topic in lymphoma research recently.
The recommendations now go to the European Commission (EC), who make a final decision on whether these treatments can be marketed throughout Europe.
NICE are already assessing both Yescarta and Kymriah to decide whether they should be funded on the NHS in the UK.
What are CAR T cells?
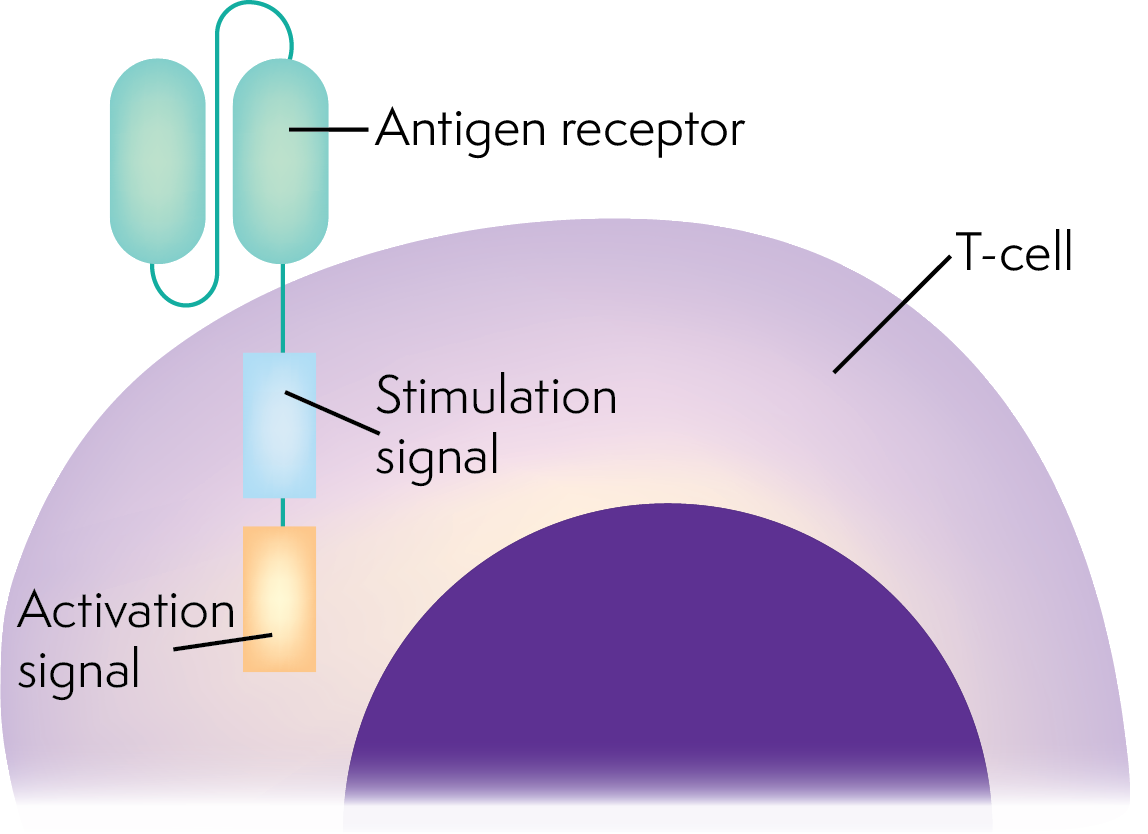
Chimeric antigen receptor T (CAR T) cell therapy uses your own immune system to destroy lymphoma cells. Your own T cells (a type of white blood cell that fights infection) can be genetically modified (changed) to recognise and kill lymphoma cells with a certain protein on their surface.
Your T cells are collected from your blood and genetically modified in a laboratory. The genetically modified T cells (CAR T-cells) are grown in the laboratory until there are enough of them, then given back to you.
