New treatment for mycosis fungoides or Sézary syndrome (SS)
Published on: 17 January 2019Mogamulizumab licensed for the treatment of mycosis fungoides or Sézary syndrome (SS)
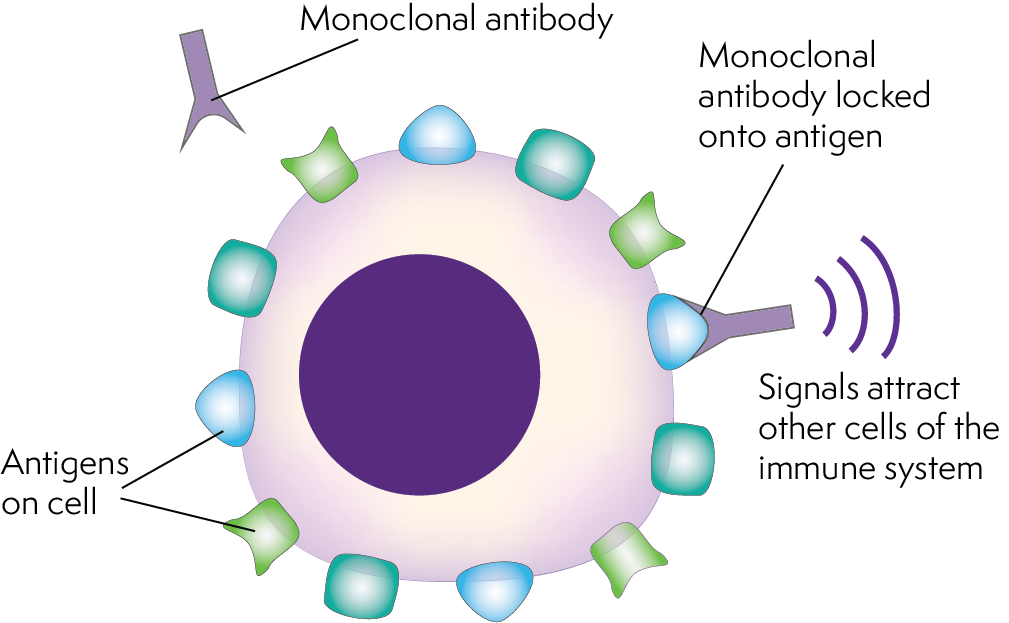
The European Commission has recently announced that mogamulizumab (brand name Poteligeo®) has been granted a licence for the treatment of adult patients with mycosis fungoides or Sézary syndrome who have received at least one previous systemic therapy.
Mycosis fungoides and Sézary syndrome are the two most common subtypes of cutaneous T-cell lymphoma, a rare type of non-Hodgkin lymphoma affecting the skin.
Mogamulizumab is an antibody therapy that targets a protein called CC chemokine receptor (CCR4). This protein is often present on cutaneous T-cell lymphoma cells.
The treatment will now be assessed by the National Institute for Health and Care Excellence (NICE) to decide if it is eligible for funding on the NHS. The appraisal process for mogamulizumab is expected to begin in early July 2019.
Lymphoma Action will publish any decisions or updates as-and-when they are available.
